"Type 1 diabetes: Integrated sensor-augmented pump therapy systems for managing blood glucose levels (The MiniMed Paradigm Veo System and the Vibe and G4 PLATINUM CGM system)"
It's not good reading.
This really knocked the wind out of my sails today - compounded by today's news reminding us that NHS is in a very precarious financial position (not that those of us in it need any reminding - my first meeting this morning was a budget meeting...).
So here are the key recommendations:-
 |
| https://www.nice.org.uk/guidance/gid-dt22/resources/type-1-diabetes-managing-blood-glucose-levels-the-minimed-paradigm-veo-system-and-the-vibe-and-g4-platinum-cgm-system-consultation-document2 |
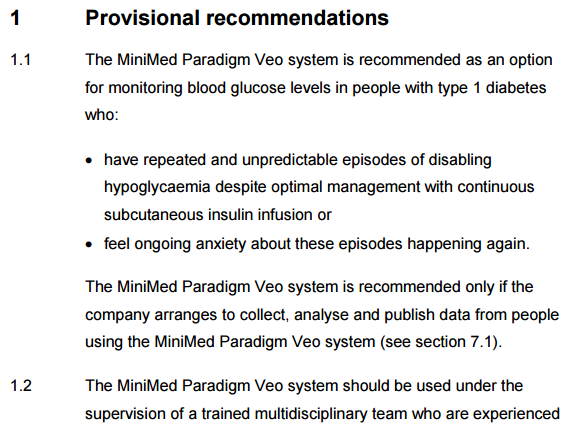
The Committee recommended that the company collect data on people using the MiniMed Paradigm Veo system, which should be analysed and published to show the system’s impact on improving control of disabling hypoglycaemia. Key outcomes that could be presented include frequency and duration of hypoglycaemic events, time spent in hypoglycaemia, and number and duration of low glucose suspend events.
In other words, Medtronic, it appears, have to publish their CareLink data, or at least a subsection of it? That's my reading of it (could be wrong of course and we're just looking at another small trial request...)
The document is hardly a ringing endorsement of CGM use... What went wrong? Yes, some of it is out of date (the 640g is yet to feature); and yes, there are some apparent factual errors in the document. But the key issue appears to be insufficient evidence, both in terms of raw statistics (numbers of subjects and data-points) and good quality, unbiased, randomised controlled trials.
NICE - and most switched-on commissioners in the NHS - want to see good quality evidence, from which clear outcomes can be extracted and a clear (financial and health) 'win' is proven for a given treatment or intervention.The Oxford Centre for Evidence-Based Medicine produce a nice table of different levels of evidence here:
We need more Level 1 and 2 data when it comes to CGM and combined Pump + CGM systems. Ideally that data to be stratified into key T1D groups (paediatrics, adults, hypo awareness etc) and over a sufficiently long period. Reliable projections of how relatively small improvements in HbA1C, for example, translate into lifetime healthcare savings also appear thin on the ground.
I've faced similar issues over the years in the day job with treatments for some less common cancers: studies have been too small, methodologies have not been consistent (i.e. you can't bolt the results together reliably). Case studies, anecdotal evidence, patient feedback can be overwhelmingly positive, but it's not enough to convince most commissioners most of the time (and also, sometimes, the Multi-Disciplinary Team meetings looking after individual patients). Of course, in this case, it's not just a case of potentially denying a treatment option, there's the very real risk of exposing the patient to significant harm by prescribing an unproven treatment in their position.
It's taken years of pushing to start to get commissioners, pharma, scientists and oncologists to begin to agree common standards. These evolving standards cover trial design, methodology of measuring and collecting parameters and monitoring outcome. It's taken commitment and good will from all parties and it's not complete yet, but it should enable 1+1=2 and evidence collection to expand quickly and reliably . Ironically it appears that the specialist commissioning shortfall that the NHS is currently facing has helped push this process along. In cancer there is huge pressure from charities and media to "do something". Type 1 Diabetes doesn't have that same voice (sorry JDRF and others, you're not on the same stage as the main cancer charities). If it did, it would have access to something like the time-limited Cancer Drugs Fund and Commissioning Through Evaluation, where rules can be loosened and a genuine effort made to help patients whilst building some of the evidence base.
In cancer, there is also a commitment to extend a national database to include these treatments - and that brings me to the elephant in the room. Diabetes data is not complicated (some of the clinical outcomes are of course), but logging and collating the stream of SG data, insulin boluses etc is straightforward. And yet, and yet, agreeing to a common standard in data structure, let alone database structure is beyond the wit of the main commercial and charity players. It's about time they looked over their shoulders to other areas of healthcare and realised that common standards not only help drive innovation, but they can help deliver the evidence base that healthcare commissioners require before parting with their increasingly rationed pot of gold.
NICE are receive comments until 25th August 2015.

No comments:
Post a Comment